Abstract
BACKGROUND AND AIMS
Allogeneic stem cell transplantation (HSCT) offers the only chance of cure for patients with advanced acute myeloid leukemia (AML). High levels of pre-HSCT minimal residual disease (MRD) have been reported to predict relapse risk after HSCT even in patient transplanted in first complete remission (CR). WT1 expression levels and multicolor flow cytometry (MFC) are the most common tools to evaluate MRD. The aim of this study was to analyze the role of pre-HSCT combined MRD assessment as predictor for the post-transplant relapse risk in patient transplanted beyond first CR.
MATERIALS AND METHODS
We retrospectively analyzed the outcome of 92 consecutive AML patients receiving HSCT in 2nd or 3rd CR. Pre-HSCT marrow samples were analyzed for WT1 expression and MFC as MRD evaluation. Median age at transplant was 45 years. Disease phase was CR2 in 63 (68%) and CR3 in 29 patients (32%). ELN risk category at diagnosis was low in 28 (30%), intermediate in 44 (48%) and high in 20 (22%) patients. Sixty-six (71%) patients received myeloablative conditioning, whereas 26 patients (29%) received reduced intensity conditioning. Stem cell source was bone marrow in most cases and donor was an HLA-identical sibling in 18 (20%), haploidentical sibling (HAPLO) in 24 (26%) and unrelated donor in 50 (54%). Median follow-up was 64 months (95% CI 39.8 - 88.2 months).
A positive MFC MRD was defined by the presence of at least 1x10-3 residual leukemic cells by 4 or 8 (since 2011) color flow-cytometry. WT1 copy number/Abl copy number 250x104 was used as cut-off value for abnormal WT1 expression.
RESULTS
Relapse occurred in 30 patients (33%) and two years non-relapse mortality was 29%. Three-year estimate of OS was 47.9% (median 19 months). The survival probability was significantly affected by donor source (better for HAPLO, p<0.05), ELN risk at diagnosis (better for ELN low risk, p<0.01), MRD status before HSCT measured with any method (p <0.01 for WT1-based MRD, p<0.03 for MFC based MRD) and CR status at HSCT (better for CR2, p<0.05).
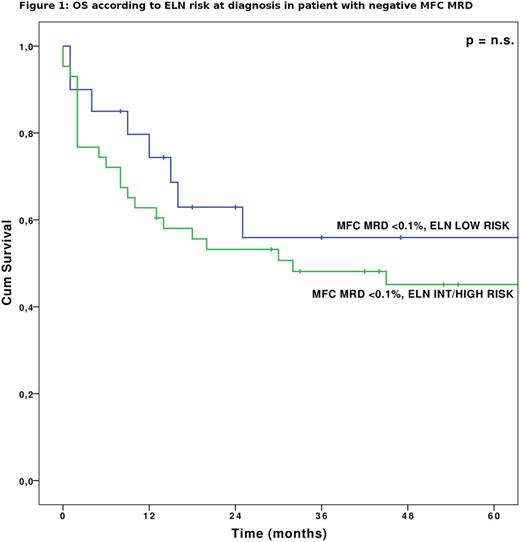
Specifically, among MRD negative patient a relatively low rate of relapse was observed regardless of risk at diagnosis (2-years OS of 62.2% and 52.7% among MFC MRD negative patient with ELN risk low or intermediate/high, respectively, Fig.1).The predictive value of MRD resulted independent from all other analyzed variables. However, patients with positive MRD did slightly better when undergoing HAPLO HSCT. Multivariate OS analysis revealed that the MRD evaluation by any method was the only independent predictor of OS (p <0.05 for both methods of analysis).
MRD evaluation was also a strong predictor of cumulative incidence (CI) of relapse in competitive risk analysis (p<0.01 and p<0.03, respectively, for WT1 and MFC MRD). Multivariate CI of relapse analysis showed that donor source and MRD evaluation significantly influenced relapse risk (p <0.05 and <0.01, respectively).
CONCLUSIONS
Pre transplant MRD evaluation by both WT1 and MFCon bone marrow samples is a reliable predictor of relapse risk which may overcome the prediction based on ELN risk at diagnosis. Both methods were able to efficiently predict post-transplant disease reoccurrence risk: patients with negative pre-HSCT MRD markers have a significantly better OS, while patients with positive MRD markers show an higher risk of relapse, irrespective of lower or higher ELN risk at diagnosis.
Furthermore, our results seem to suggest that HAPLO donor may have a positive impact in reducing relapse rate in advanced AML patients, in particular in patients with positive MRD.
Refining the baseline risk assessment based on ELN risk in patient undergoing HSCT beyond CR1 who have an higher risk of relapse is very important when evaluating optimal stem cell source and conditioning intensity and could allow to apply pre-emptive therapeutic strategies to prevent AML relapse, from donor lymphocyte infusion to other innovative approaches.
Angelucci: Roche: Other: Advisory board: biosimilars; Novartis Oncology: Other: Protocol Telesto: sterring committee Chair; Bluebird Bio: Other: Advisory board: Gene therapy in Thalassemia; Jazz: Other: Advisory board: AML; Celgene: Other: protocoll ACE 536 B-Thal 001: DMC Chair; Novartis Oncology: Other: Advisory board: iron toxicity; Novartis Oncology Swiss: Other: Invited speakers sponsored satellite meeting during ; Celgene: Honoraria, Other: Advisory: research project .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal